Polar Encyclopædia
THE GREENHOUSE EFFECT

LIKE A GLASSHOUSE AROUND THE GLOBE
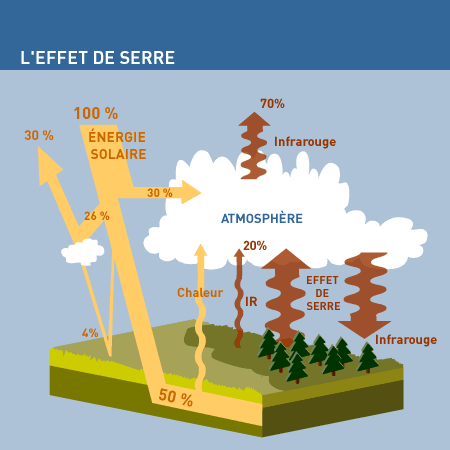
Given the distance between the Earth and the Sun, our planet should be icy cold (-18°C), but the average temperature at the Earth’s surface is actually +15°C. Why? Because the atmosphere around the Earth acts like a greenhouse, letting the Sun’s rays in to warm us up and preventing the heat from escaping again. This phenomenon is called the “greenhouse effect”.
GASES IN TINY AMOUNTS BUT PLAYING A VITAL ROLE
The water vapour present in the air accounts for two thirds of the greenhouse effect. But there are gases, sometimes present in very small amounts, that also contribute to it. The main greenhouse gases, or GHG, are: carbon dioxide (CO2), methane, nitrogen protoxide, ozone (a sort of super-oxygen found in the lower atmosphere) and compounds called CFCs.
AN AGE-OLD HEATING SYSTEM
Right from the time our solar system was formed, 4.6 billion years ago, the greenhouse effect has regulated the Earth’s climate. But the atmosphere has evolved quite a lot since its original and primitive composition. One marked change was that photosynthesis, starting with the earliest plants, gradually lowered the proportion of CO2 in the atmosphere, helping to maintain a life-friendly temperature on Earth.

A PHENOMENON AMPLIFIED BY HUMAN ACTIVITY
In its original state, the greenhouse effect was a completely natural phenomenon and invaluable to the Earth’s equilibrium. But the effect can be amplified if the amounts of GHG present are allowed to increase. And this is now happening as a result of the pollution generated by human activities. This is raising concerns about possible climate problems in future.
THE MAIN FACTORS BEHIND THE GREENHOUSE EFFECT ARE :
> Water vapour (H20), which is produced by evaporation of the water in plants, soil, watercourses and the ocean. A small amount is also emitted by volcanoes (this is how the planet’s original, primitive atmosphere was formed).
> Carbon dioxide (CO2), which is a natural gas, is generated by: the breathing process of living beings (while the photosynthesis of green plants consumes CO2); combustion (forest fires); and volcanic eruptions. The amount of CO2 in the atmosphere is regulated by the ocean, which can absorb large quantities of the gas.
> Methane (CH4) is also a natural gas. It is given off by the decomposition of organic matter where no oxygen is present (rotting in marshes, mangroves, thawed ground in the Arctic and of course digestion in animal stomachs). It is also given off when volcanoes erupt.
> Ozone (O3), present in the lower atmosphere (troposphere), is a natural gas too. It probably descends from the stratosphere via “pockets” punched by the upper jet stream. Ozone is like oxygen except that is has three oxygen atoms instead of two. The gas is a veritable poison to plants as well as human skin and our respiratory system (asthma attacks). But at altitude, say 20 or 30 km up, ozone is a very useful gas, as it protects us against ultraviolet solar radiation, which is dangerous to most life forms. This is the gas referred to when ecologists talk of the seasonal hole in the ozone layer, first discovered overhead the Antarctic and now reckoned to be diminishing – a phenomenon that worries scientists because of the potential dangers to human beings.
> Nitrogen protoxide is one of the substances produced when nitrates in the soil are broken down by bacteria. It then escapes into the atmosphere and reacts to produce nitrogen oxide (NO).
> CFCs or chloro-fluoro-carbons, are produced as a result of human activities. They are mentioned here so as to be compared with other GHGs.
THE IMPORTANCE OF GREENHOUSE GASES :
| Gas | Concentration (ppm*) | Greenhouse effect efficiency | Contribution to the greenhouse effect (Watt/m2) |
| H2O (water vapour) | 3 000 | 1NBSP. | 100 |
| CO2 | 355 | 1 | 50 |
| CH4 | 1.8 | 21 | 1.8 |
| N20 | 0.3 | 310 | 1.3 |
| 03 | < 0.1 | 2 000 | 1.3 |
| CFC | 0.01 | 10 000 | 0.3 |
* ppm = parts per million, i.e. 1cm3 per m3 of air
PHYSICIST’S CORNER
GHGs are present in the atmosphere in only minute amounts, and these amounts usually fluctuate too. However, the molecules of these gases, formed of two or more atoms, vibrate and become agitated when struck by the infrared radiation emitted outwards by the land or ocean, and this heats up the atmosphere so that it becomes a huge “heat trap” producing energy at a rate of 340 W/m2, much more than the amount of solar radiation directly striking the Earth’s surface (158 W/m2).
When the atmospheric variations speed up of their own accord we speak of “positive retro-action”, and when they slow down it is called “negative retro-action”. It is the water vapour in the atmosphere that amplifies the variations in the greenhouse effect. In accordance with the laws of physics (in this case Clausius-Clapeyron’s Law), the colder air becomes the less water vapour it can hold (hence the formation of fog in cold weather), and this diminishes the greenhouse effect. On the other hand, the warmer the air, the more water vapour it can hold (hot, humid tropical climates), and this increases the greenhouse effect.
If, at a given pressure and a temperature of -20°C, 1 kg of air can hold 1 g of water in vapour state, at -10°C it can hold 2 g. But at +20°C, the same volume of air can hold 15 g of water vapour, and 26 g at +30°C. Beyond this point, the air is saturated and the vapour will form water droplets or ice crystals, resulting in fog, frost, clouds or condensation mist on objects.

JEAN-LOUIS ETIENNE AND MEASUREMENT OF GAS EMISSIONS
During the EREBUS mission, Jean-Louis Etienne and his companions took atmospheric samples of trace gases (ozone, methane, etc.) throughout the voyage of the “Antarctica” down towards the southernmost continent. These measurements formed part of a study programme to find out more about the complex chemistry of trace gases in the atmosphere. This “photo-chemistry”, so called because it is affected by the sun, regulates the delicate but little-known balances in the Earth’s atmosphere.

DID YOU KNOW ?
The Moon has no atmosphere at all because its mass is too low to retain gases around it, so the Moon has no greenhouse effect. Venus and Mars, on the other hand, do have dense atmosphere and the greenhouse effect is present.
TO FIND OUT MORE …
BIBLIOGRAPHY
- L’effet de serre (Numéro spécial, La Recherche n° 243, 1992)
- Dossier pédagogique : L’Arctique et l’environnement boréal (P. Avérous CNDP, 1995)
- Expédition Erebus (J.-L. Etienne / P. Avérous- Arthaud-1994)
- Dossier pédagogique “EREBUS” : L’environnement polaire 2 (P. Avérous-Autrement dit, CNDP-1994)
- Encyclopédia Universalis
- La Terre… notre planète (P. Avérous-Nathan-1990)
- Atmosphère, Atmosphère (Science & Vie, Hors série n° 174-1991)
- Quel climat pour demain ? (S. Huet, Calmann-Levy, 2000)
- L’incertitude des climats (R. Kandel, Hachette, 1998)
- Le climat de la Terre (R. Sadourny, DOMINOS, Flammarion, 1994)
Support the project with a donation
The Polar POD expedition is one of the stamp of the pioners, a human adventure coupled with a technological challenge, an oceanographic exploration never before carried out which will mark a milestone in the discovery of the oceans.
Thank you for your support !